Abstract
Background:
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening thrombotic microangiopathy (TMA) occurring due to an acquired deficiency in ADAMTS13. Mortality due to iTTP is estimated at 10% with current standard treatment that consists of plasma exchange (PLEX) and corticosteroids. The United States TMA (USTMA) registry incorporates 15 large US referral centers across the nation, and includes patients diagnosed with iTTP between 1985 and 2019. We sought to perform a descriptive analysis on the patients with fatal outcomes attributable to acute iTTP episodes in the registry.
Methods:
We utilized the USTMA registry (n=771) and analyzed twenty-two baseline patient demographics, presenting symptoms, and laboratory findings. The study cohort included participants with iTTP diagnosis based on the presence of thrombocytopenia (platelet count <100 /µL), microangiopathic hemolyic anemia (hemoglobin less than the lower limit of normal with schistocytes on the peripheral blood smear), and either ADAMTS13 activity <10% or ADAMTS13 activity <20% with an anti-ADAMTS13 inhibitor or antibody. For participants diagnosed before the ADAMTS13 assay was developed (2006), the iTTP diagnosis was based on the clinical course and absence of alternative causes. iTTP exacerbation was defined as clinical disease recurrence within 30 days of PLEX discontinuation, and clinical relapse was defined as disease recurrence after 30 days of last PLEX, as per the international working group definitions 1.
Results:
A total of 33 patients (4.28%) in the USTMA cohort died during acute iTTP episodes. The patient demographics and initial presenting lab values are summarized in Table 1.
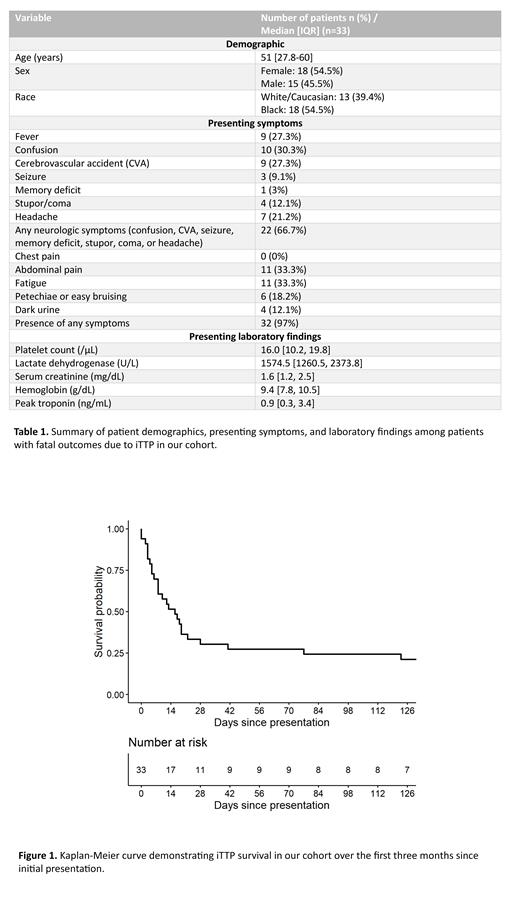
Time of death (Figure 1):
22 patients (66.7%) died during the initial iTTP episode, and within 30 days of presentation.
3 patients (9.0%) died during disease exacerbation of the initial episode.
8 patients (24.2%) died due to disease relapse.
Median time to death at initial presentation/exacerbation (n=25) = 8 days [IQR: 4-19]
Median time to death due to relapse = 1.6 years [IQR: 1.1-5.7]
Patient demographics and presenting features (Table 1):
Median age = 51 years [IQR: 27.8-60]
Sex = 54.5% female, 45.5% male
Presence of neurologic symptoms on presentation: 22 (66.7%)
Presence of any symptoms on presentation: 32 (97%)
Conclusion:
Patients with fatal outcomes due to acute iTTP episodes presented with variable symptoms and baseline characteristics. While the vast majority of deaths occurred during the initial acute episode, death also occurred during exacerbation of the initial episode or subsequent disease relapse. Vigilant laboratory and clinical monitoring both after achieving initial remission and during long-term follow-up are necessary, to allow detection of disease exacerbation and relapse, and potentially prevent iTTP-related deaths.
1. Cuker A, Cataland SR, Coppo P, et al. Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021;137(14):1855-1861.
Mazepa: Answering TTP Foundation: Research Funding; Sanofi Aventis: Other. Lim: Hema Biologics: Honoraria; Sanofi Genzyme: Honoraria; Dova Pharmaceuticals: Honoraria.